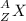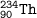Chemistry, 14.12.2020 09:50 barb4you67
The symbol for a radioactive nuclide is Superscript 234 subscript 90 upper T h.. Which statement is correct?
The atomic number of the radioactive nuclide is 90.
The mass number of the radioactive nuclide is 144.
The number of neutrons that are present in the nucleus of each atom is 324.
The number of protons that are present in the nucleus of each atom is 234.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
The symbol for a radioactive nuclide is Superscript 234 subscript 90 upper T h.. Which statement is...
Questions








Biology, 22.08.2020 04:01





Mathematics, 22.08.2020 04:01


Mathematics, 22.08.2020 04:01

Advanced Placement (AP), 22.08.2020 04:01

Mathematics, 22.08.2020 04:01

History, 22.08.2020 04:01

Physics, 22.08.2020 04:01