
Chemistry, 14.12.2020 18:20 research73
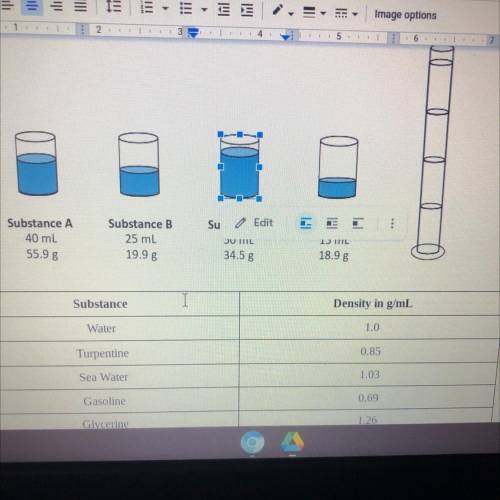
A chemistry class is given four beakers containing unknown liquids A, B, C, and shown below.
Address each of the following prompts in a one page essay below.
• Predict what would happen if liquids A, B,C, and D were added to the graduated cylinder
and use quantitative data as evidence to support your prediction.
• Describe the graduated cylinder with the names of each substance and the order in
which they would occur based on their densities.
• Finally, predict where a sphere with a mass of 2.5g and a volume of 2.33 cm would stop
in the column. Justify your reasoning with quantitative data.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
A chemistry class is given four beakers containing unknown liquids A, B, C, and shown below.
Addres...
Questions

Computers and Technology, 28.01.2020 07:31


Mathematics, 28.01.2020 07:31

History, 28.01.2020 07:31






English, 28.01.2020 07:31


Social Studies, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31

English, 28.01.2020 07:31


Computers and Technology, 28.01.2020 07:31



Mathematics, 28.01.2020 07:31



