If you have 116 grams of Table Salt (NaCl), how many moles of salt do you have?
a
Ob
1....

Chemistry, 14.12.2020 21:40 smariedegray
If you have 116 grams of Table Salt (NaCl), how many moles of salt do you have?
a
Ob
1.99 g
3.27 g
5.05 g
0.50 g
d
Question 8 point

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
Questions



History, 24.01.2020 00:31

Mathematics, 24.01.2020 00:31

Mathematics, 24.01.2020 00:31





Mathematics, 24.01.2020 00:31






Mathematics, 24.01.2020 00:31



English, 24.01.2020 00:31

Spanish, 24.01.2020 00:31

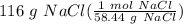 = 1.98494 mol NaCl
= 1.98494 mol NaCl

