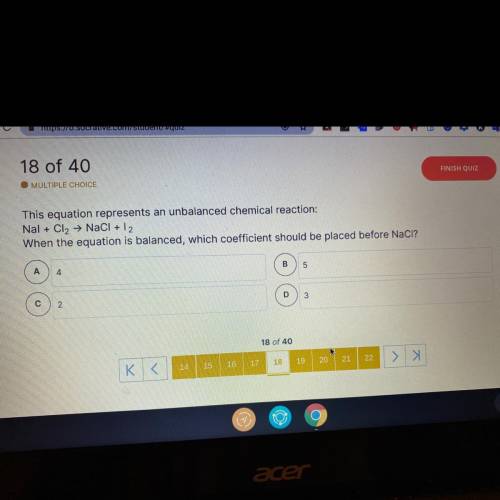
This equation represents an unbalanced chemical reaction:
Nal + Cl2 → NaCl + 12
When the equa...

Chemistry, 15.12.2020 05:40 startabull
This equation represents an unbalanced chemical reaction:
Nal + Cl2 → NaCl + 12
When the equation is balanced, which coefficient should be placed before NaCl?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Questions

Mathematics, 27.03.2020 22:55

Mathematics, 27.03.2020 22:55




Social Studies, 27.03.2020 22:55

History, 27.03.2020 22:55


Mathematics, 27.03.2020 22:55

History, 27.03.2020 22:55

Mathematics, 27.03.2020 22:56



English, 27.03.2020 22:56

Biology, 27.03.2020 22:56

English, 27.03.2020 22:56






