
Chemistry, 15.12.2020 09:00 bcarri4073
QuesLIVII 2 PUNILS)
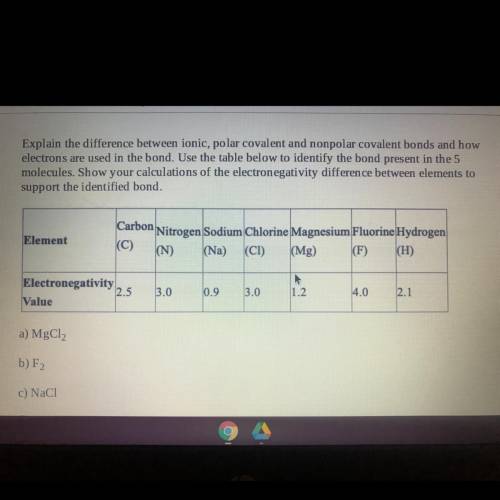
Explain the difference between ionic, polar covalent and nonpolar covalent bonds and how
electrons are used in the bond. Use the table below to identify the bond present in the 5
molecules. Show your calculations of the electronegativity difference between elements to
support the identified bond.
Element
Carbon
Nitrogen Sodium Chlorine Magnesium Fluorine Hydrogen
(N)
(Mg) (F) (H)
(Na)
(CI)
Electronegativity
2.5
Value
3.0
0.9
3.0
1.2
4.0
2.1
a) MgCl2
b) F2
c) NaCl
Sign Out

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 08:00
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3
You know the right answer?
QuesLIVII 2 PUNILS)
Explain the difference between ionic, polar covalent and nonpolar covalent bond...
Questions



History, 21.01.2022 02:20

Chemistry, 21.01.2022 02:20

History, 21.01.2022 02:20



English, 21.01.2022 02:20

Mathematics, 21.01.2022 02:20

Biology, 21.01.2022 02:20

Mathematics, 21.01.2022 02:20


English, 21.01.2022 02:20

Mathematics, 21.01.2022 02:30


History, 21.01.2022 02:30


Mathematics, 21.01.2022 02:30

Social Studies, 21.01.2022 02:30



