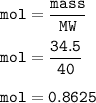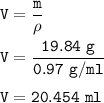Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1


Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
How many milliliters of sodium metal, with a density of 0.97 g/mL, would be needed to produce 34.5 g...
Questions


History, 16.12.2020 18:00

Computers and Technology, 16.12.2020 18:00


Mathematics, 16.12.2020 18:00

Mathematics, 16.12.2020 18:00


Biology, 16.12.2020 18:00






Mathematics, 16.12.2020 18:00




Health, 16.12.2020 18:00

History, 16.12.2020 18:00

Mathematics, 16.12.2020 18:00