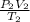Chemistry, 15.12.2020 09:30 confi92841
A sample of gas in a balloon has an initial temperature of 42 ∘C and a volume of 1.10×103 L . If the temperature changes to 86 ∘C , and there is no change of pressure or amount of gas, what is the new volume, V2, of the gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2


Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3
You know the right answer?
A sample of gas in a balloon has an initial temperature of 42 ∘C and a volume of 1.10×103 L . If the...
Questions


English, 23.02.2021 07:10




Mathematics, 23.02.2021 07:10


Arts, 23.02.2021 07:10

Mathematics, 23.02.2021 07:10


Mathematics, 23.02.2021 07:10

Spanish, 23.02.2021 07:10



Chemistry, 23.02.2021 07:10



SAT, 23.02.2021 07:10


History, 23.02.2021 07:10

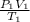 =
=