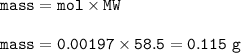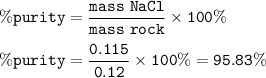0.12g of rock salt was dissolved in water and titrated with 0.1moldm^-3 silver nitrate until the first permanent brown precipitate of silver chromate is seen. 19.70 cm^3 was required to titrate all the chloride ion. how many moles of chloride ion were titrated? what mass of sodium chloride was titrated? what was the % purity of the rock salt in terms of sodium chloride?

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1

Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1

Chemistry, 23.06.2019 11:30
The dashed segment of the plotted experiment in the graph in the l
Answers: 3
You know the right answer?
0.12g of rock salt was dissolved in water and titrated with 0.1moldm^-3 silver nitrate until the fir...
Questions

English, 06.04.2021 23:20

Mathematics, 06.04.2021 23:20

Mathematics, 06.04.2021 23:20

Business, 06.04.2021 23:20




Mathematics, 06.04.2021 23:20



Mathematics, 06.04.2021 23:20


Mathematics, 06.04.2021 23:20



Chemistry, 06.04.2021 23:20

Chemistry, 06.04.2021 23:20



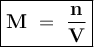
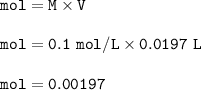 .
.