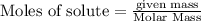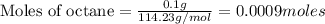Chemistry, 15.12.2020 16:40 BlueExorcistReaper
The enthalpy of combustion of octane is -5470 kJ/mol. Octane (formulae C8H18) was used to heat some water in a copper can. The amount of octane used up was 0.1 g. The amount of water in the can was 100 cm3 . a) Find the moles of octane which was used up. (Show calculations)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 09:30
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3
You know the right answer?
The enthalpy of combustion of octane is -5470 kJ/mol. Octane (formulae C8H18) was used to heat some...
Questions




Mathematics, 17.07.2019 10:10

Mathematics, 17.07.2019 10:10



English, 17.07.2019 10:10



Biology, 17.07.2019 10:10


Social Studies, 17.07.2019 10:10

Mathematics, 17.07.2019 10:10

Mathematics, 17.07.2019 10:10



Social Studies, 17.07.2019 10:10


English, 17.07.2019 10:10