
Chemistry, 15.12.2020 16:50 sherry59Sherry59
Calculate the amounts of each substance in the reaction below if an initial amount of 0.400 moles are brought together with an initial amount of 2.29 moles of Cl2 in a 1.00 L vessel

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Calculate the amounts of each substance in the reaction below if an initial amount of 0.400 moles ar...
Questions

Mathematics, 26.01.2021 04:10

History, 26.01.2021 04:10

Mathematics, 26.01.2021 04:10

Mathematics, 26.01.2021 04:10




Mathematics, 26.01.2021 04:10



Health, 26.01.2021 04:10

History, 26.01.2021 04:10

Mathematics, 26.01.2021 04:10

History, 26.01.2021 04:10


History, 26.01.2021 04:10

Mathematics, 26.01.2021 04:10



Mathematics, 26.01.2021 04:10

![[COCl_2 ] = 0.282 \ M](/tpl/images/0985/2587/8cecc.png)
![[CO] = 0.12 \ M](/tpl/images/0985/2587/06beb.png)
![[Cl_2] = 2.008 \ M](/tpl/images/0985/2587/62ed3.png)
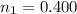
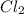 is
is 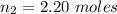
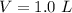
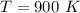
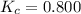
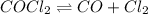
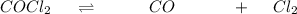
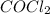 and
and 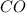 and
and ![K_c = \frac{[CO] [Cl_2]}{[COCl_2]}](/tpl/images/0985/2587/df366.png)
![0.800 = \frac{[0.400 - x] [2.20 - x ]}{[x]}](/tpl/images/0985/2587/ca900.png)
![0.800 x = [0.400 - x ] * [ 2.20 - x ]](/tpl/images/0985/2587/18466.png)
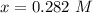
![[CO] = 0.400 - 0.282](/tpl/images/0985/2587/23af3.png)
![[Cl_2 ] = 2.29 - 0.282](/tpl/images/0985/2587/2fc18.png)


