Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
You know the right answer?
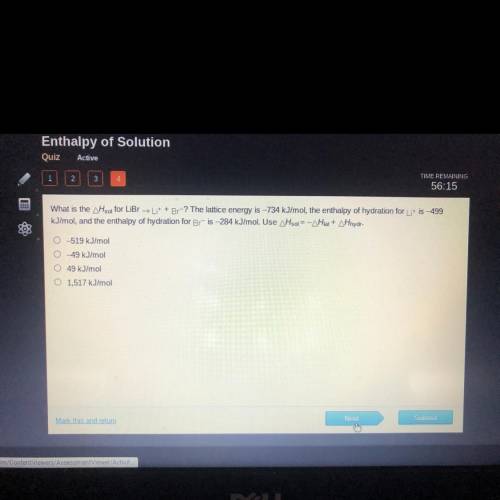
What is the AH sol for LiBr → Li+ + Br-? The lattice energy is -734 kJ/mol, the enthalpy of hydratio...
Questions


Social Studies, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00




Social Studies, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00

Geography, 14.09.2021 14:00



Mathematics, 14.09.2021 14:00




Arts, 14.09.2021 14:00

Geography, 14.09.2021 14:00

English, 14.09.2021 14:00

Mathematics, 14.09.2021 14:00