
Chemistry, 15.12.2020 22:20 kendramiller3965
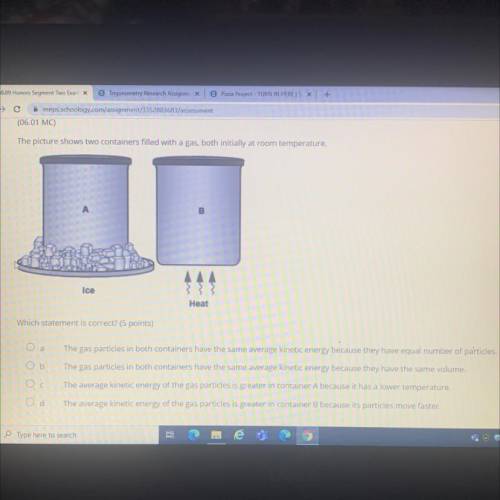
The picture shows two containers filled with a gas, both initially at room temperature.
B
Ice
Heat
Which statement is correct? (5 points)
О а
The gas particles in both containers have the same average kinetic energy because they have equal number of particles.
Ob
The gas particles in both containers have the same average kinetic energy because they have the same volume.
Ос
The average kinetic energy of the gas particles is greater in container A because it has a lower temperature.
The average kinetic energy of the gas particles is greater in container B because its particles move faster.
Od

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1


Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
The picture shows two containers filled with a gas, both initially at room temperature.
B
Ice...
Ice...
Questions

Biology, 26.06.2019 06:00



Mathematics, 26.06.2019 06:00

History, 26.06.2019 06:00



English, 26.06.2019 06:00

History, 26.06.2019 06:00


Biology, 26.06.2019 06:00

Mathematics, 26.06.2019 06:00

Mathematics, 26.06.2019 06:00


English, 26.06.2019 06:00

History, 26.06.2019 06:00

Mathematics, 26.06.2019 06:00

Mathematics, 26.06.2019 06:00




