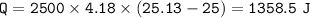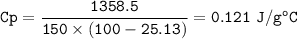Chemistry, 16.12.2020 08:10 kierafisher05
The murder weapon weighs 150 grams. The initial temperature of the weapon before being tested was 100 oC. After placing the weapon in the water, the temperature dropped to 25.13 oC. Find the specific heat capacity of the unknown metal by first calculating the heat energy gained by the water.
Water
Mass = 2500 g
Initial temperature = 25 o C Final temperature = 25.13 oC
Equations
q = m C p Δ T C p = q / (mΔT)
1. How much heat energy (q) did the water gain?
2. What is the specific heat capacity (C p) of the unknown metal?
3. Compare the heat capacity you calculated with the one below, which one matches?
Specific heat capacity of metal (J / g or C)
Silver 0.235
Gold 0.129 Lead 0.121
Copper
4. What metal was the murder weapon made of?
0.385

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
The murder weapon weighs 150 grams. The initial temperature of the weapon before being tested was 10...
Questions

Physics, 23.12.2019 22:31


Social Studies, 23.12.2019 22:31

Mathematics, 23.12.2019 22:31



Mathematics, 23.12.2019 22:31



Mathematics, 23.12.2019 22:31

Mathematics, 23.12.2019 22:31

Mathematics, 23.12.2019 22:31

English, 23.12.2019 22:31


Mathematics, 23.12.2019 22:31