
Chemistry, 16.12.2020 14:00 lesliealaves16
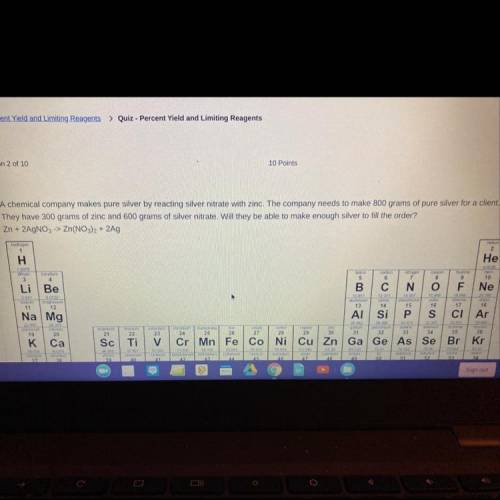
A chemical company makes pure silver by reacting silver nitrate with zinc. The company needs to make s00 grams of pure silver for a client.
They have 300 grams of zinc and 600 grams of silver nitrate. Will they be able to make enough silver to fil the order?
Zn + 2AgNO,> Zn(NO), + 2Ag A. Yes, there will be extra left over. B. Yes, they will have exactly enough C. No, the silver nitrate will run out first. D. No, the zinc nitrate will run out first

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
A chemical company makes pure silver by reacting silver nitrate with zinc. The company needs to make...
Questions


Chemistry, 01.10.2019 23:30


Mathematics, 01.10.2019 23:30






Biology, 01.10.2019 23:30









English, 01.10.2019 23:30

Computers and Technology, 01.10.2019 23:30



