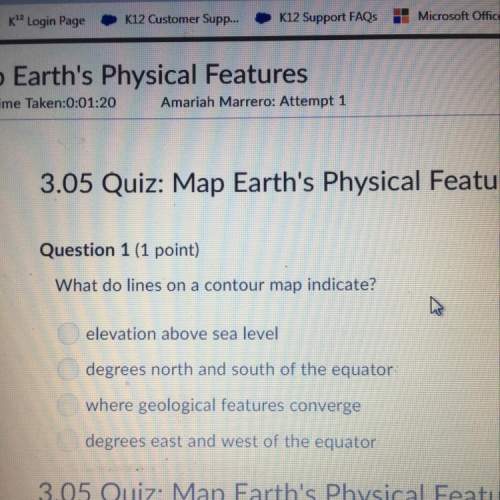
Chemistry, 16.12.2020 17:00 gregorio03
A student performed a titration experiment to determine the molarity of a sodium hydroxide solution. 22.45 mL of a NaOH solution were required to reach the equivalence point when titrating 50.0 mL of a solution containing 2.4567 g of potassium hydrogen phthalate (KHC8H4O4). What is the concentration of the sodium hydroxide solution?
a. 0.2406 M NaOH
b. 0.5358 M NaOH
c. 0.1094 M NaOH
d. 1.782 M NaOH
e. none of these

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

You know the right answer?
A student performed a titration experiment to determine the molarity of a sodium hydroxide solution....
Questions

Mathematics, 29.01.2021 01:00

Mathematics, 29.01.2021 01:00

Advanced Placement (AP), 29.01.2021 01:00


Biology, 29.01.2021 01:00



Mathematics, 29.01.2021 01:00


Physics, 29.01.2021 01:00


Mathematics, 29.01.2021 01:00



Mathematics, 29.01.2021 01:00


Mathematics, 29.01.2021 01:00



Law, 29.01.2021 01:00