Write the net ionic equation for the reaction between MnO4- ions and H2O2 in acid solution.
...

Chemistry, 16.12.2020 23:00 JoshuaXYP9978
Write the net ionic equation for the reaction between MnO4- ions and H2O2 in acid solution.
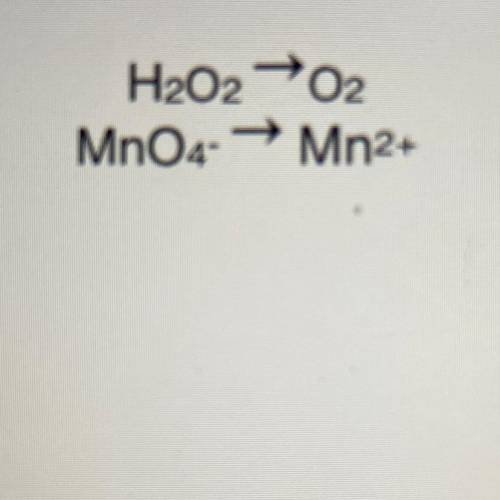

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

You know the right answer?
Questions


History, 01.08.2019 15:30

Business, 01.08.2019 15:30

Chemistry, 01.08.2019 15:30

Geography, 01.08.2019 15:30


Mathematics, 01.08.2019 15:30


Mathematics, 01.08.2019 15:30


Health, 01.08.2019 15:30

English, 01.08.2019 15:30







Mathematics, 01.08.2019 15:30

English, 01.08.2019 15:30



