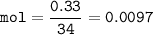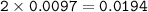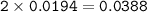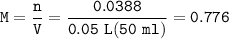Chemistry, 17.12.2020 02:30 getzperez1962
0.33 g of hydrosulfuric acid, H25, is added to 50.0 mL of water. What is the concentration (molarity) of [H301 of this solution?
H25 --> 2H +52
2H+ + 2 H20 --> 2 H30* + 2 OH
[Do not include units in your answer]

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

You know the right answer?
0.33 g of hydrosulfuric acid, H25, is added to 50.0 mL of water. What is the concentration (molarity...
Questions


Biology, 19.08.2019 12:50

Mathematics, 19.08.2019 12:50





History, 19.08.2019 12:50

Mathematics, 19.08.2019 12:50

Biology, 19.08.2019 12:50


English, 19.08.2019 12:50


Social Studies, 19.08.2019 12:50




Mathematics, 19.08.2019 12:50

Geography, 19.08.2019 12:50