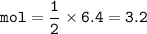Chemistry, 17.12.2020 02:40 leeney6166
Upon balancing the equation below, how many moles of sulfuric acid are needed to react completely with 6.4 moles of lithium hydroxide?
LiOH + H2SO4yields Li2SO4 + H2O (2 points)
Group of answer choices
12.8 mol H2SO4
6.4 mol H2SO4
3.2 mol H2SO4
2.1 mol H2SO4

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Upon balancing the equation below, how many moles of sulfuric acid are needed to react completely wi...
Questions




Mathematics, 08.02.2021 14:40


Mathematics, 08.02.2021 14:40

History, 08.02.2021 14:40


Computers and Technology, 08.02.2021 14:40

Mathematics, 08.02.2021 14:40

Mathematics, 08.02.2021 14:40


Business, 08.02.2021 14:40


History, 08.02.2021 14:40


Mathematics, 08.02.2021 14:40

Geography, 08.02.2021 14:40

Mathematics, 08.02.2021 14:40

Mathematics, 08.02.2021 14:40