
Chemistry, 17.12.2020 05:20 vicky10445
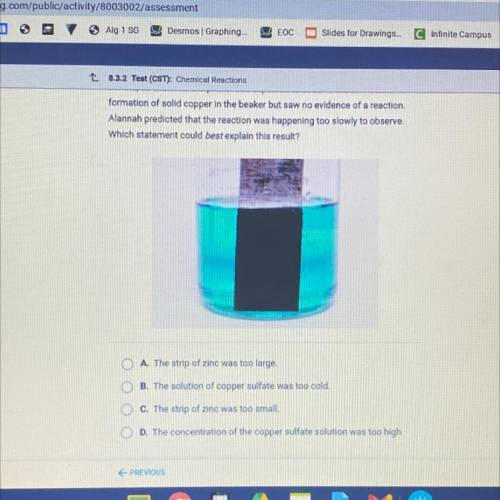
Alannah added a strip of zinc to a beaker containing a solution of copper
sulfate, as shown in the photo. She expected to observe the immediate
formation of solid copper in the beaker but saw no evidence of a reaction.
Alannah predicted that the reaction was happening too slowly to observe.
Which statement could best explain this result?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3


Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 14:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 6.00 mol fe and 8.45 mol nio(oh) react?
Answers: 1
You know the right answer?
Alannah added a strip of zinc to a beaker containing a solution of copper
sulfate, as shown in the...
Questions


Biology, 22.08.2019 18:00


History, 22.08.2019 18:00

Health, 22.08.2019 18:00


Mathematics, 22.08.2019 18:00

Mathematics, 22.08.2019 18:00

English, 22.08.2019 18:00


Mathematics, 22.08.2019 18:00

History, 22.08.2019 18:00

Mathematics, 22.08.2019 18:00

History, 22.08.2019 18:00

Chemistry, 22.08.2019 18:00


Mathematics, 22.08.2019 18:00



History, 22.08.2019 18:00



