Chemistry, 17.12.2020 09:50 xochitlbmartine
Use the reaction given below to solve the problem that follows: Calculate the mass in grams of aluminum oxide produced by the reaction of 15.0 g of aluminum metal.
[ ]grams Al2O3
4 Al + 3 O2 --> 2 Al2O3
**Your answer should be written as XX. X

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
Use the reaction given below to solve the problem that follows: Calculate the mass in grams of alumi...
Questions

Health, 29.06.2019 13:00




English, 29.06.2019 13:00



Social Studies, 29.06.2019 13:00



Mathematics, 29.06.2019 13:00

Arts, 29.06.2019 13:00



Health, 29.06.2019 13:00

Mathematics, 29.06.2019 13:00


Mathematics, 29.06.2019 13:00

Mathematics, 29.06.2019 13:00

Mathematics, 29.06.2019 13:00

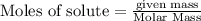
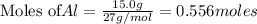
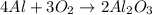
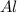 produce == 2 moles of
produce == 2 moles of 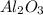
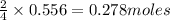 of
of