Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

You know the right answer?
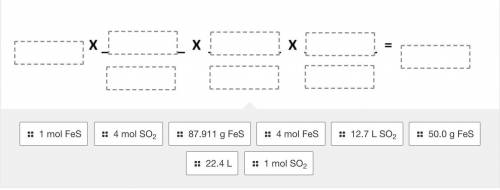
Use the balanced equation given below to drag and drop the terms to correctly solve the following pr...
Questions





Mathematics, 21.06.2019 19:30



Mathematics, 21.06.2019 19:30

Mathematics, 21.06.2019 19:30