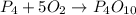P4+ __02_ P4010
I need help ASAP...

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Questions





Mathematics, 13.01.2021 18:00

Arts, 13.01.2021 18:00


Chemistry, 13.01.2021 18:00

Mathematics, 13.01.2021 18:00

Mathematics, 13.01.2021 18:00

Social Studies, 13.01.2021 18:00


Mathematics, 13.01.2021 18:00

History, 13.01.2021 18:00

History, 13.01.2021 18:00




History, 13.01.2021 18:00

Social Studies, 13.01.2021 18:00