Calculate ΔH° = for the chemical reaction
Cl2(g) + F2(g) --->2ClF(g)
Bond Energies...

Chemistry, 17.12.2020 18:00 NycJahlilThegoat
Calculate ΔH° = for the chemical reaction
Cl2(g) + F2(g) --->2ClF(g)
Bond Energies, kJ mol-1
F---F 159
Cl--Cl 243
Cl--F 255

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1


Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
You know the right answer?
Questions

History, 20.06.2021 09:30

Mathematics, 20.06.2021 09:30

English, 20.06.2021 09:30



Chemistry, 20.06.2021 09:30

Chemistry, 20.06.2021 09:30

Mathematics, 20.06.2021 09:30


Chemistry, 20.06.2021 09:40

Mathematics, 20.06.2021 09:40






Mathematics, 20.06.2021 09:40

SAT, 20.06.2021 09:40


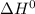 for the reaction
for the reaction 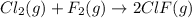 is -108kJ
is -108kJ![\Delta H=[n\times B.E_{reactants}]-[n\times B.E{products}]](/tpl/images/0995/4412/589d1.png)
![\Delta H=[1\times B.E_{Cl_2}+1\times B.E_{F_2}]-[2\times B.E_{ClF}]](/tpl/images/0995/4412/c74ae.png)
![\Delta H=[(1\times 243)+(1\times 159)]-[2\times 255]](/tpl/images/0995/4412/fbd29.png)




