
Chemistry, 17.12.2020 20:00 mikurrjurdan
The synthesis of NH3 is represented by the equation above. based on the equilibrium constant, K and delta H rxn given above, which of the following can best be used to justify that the reaction is thermodynamically favorable at 298 K and constant pressure?
N2 (g) + 3H2 (g) --> 2NH3 (g)
K= 5.6 × 10^5 at 298 K
delta H rxn= -91.8 kj/mol
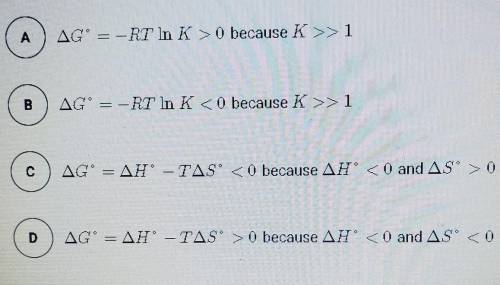

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
The synthesis of NH3 is represented by the equation above. based on the equilibrium constant, K and...
Questions



Mathematics, 10.05.2020 17:57




Advanced Placement (AP), 10.05.2020 17:57

History, 10.05.2020 17:57

Social Studies, 10.05.2020 17:57




History, 10.05.2020 17:57






English, 10.05.2020 18:57

Mathematics, 10.05.2020 18:57



