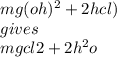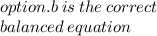Chemistry, 17.12.2020 20:40 nessuhbae6731
What is the complete balanced equation for the reaction
between magnesium hydroxide and hydrogen chloride to
produce magnesium chloride and water is
O MgOH + HCI → MgCl + H2O.
O Mg(OH)2 + 2HCl → MgCl2 + 2H20.
O Mg(OH)2 + HCl → Mg + 2CO2 +3H20.
O Mg(OH)2 + 2HCl → MgCl2 + H2 + O2.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2


You know the right answer?
What is the complete balanced equation for the reaction
between magnesium hydroxide and hydrogen ch...
Questions

Mathematics, 23.05.2021 21:40

Advanced Placement (AP), 23.05.2021 21:40


History, 23.05.2021 21:40

English, 23.05.2021 21:40



Mathematics, 23.05.2021 21:40

English, 23.05.2021 21:40


Biology, 23.05.2021 21:40


History, 23.05.2021 21:40


Mathematics, 23.05.2021 21:50



English, 23.05.2021 21:50


Mathematics, 23.05.2021 21:50