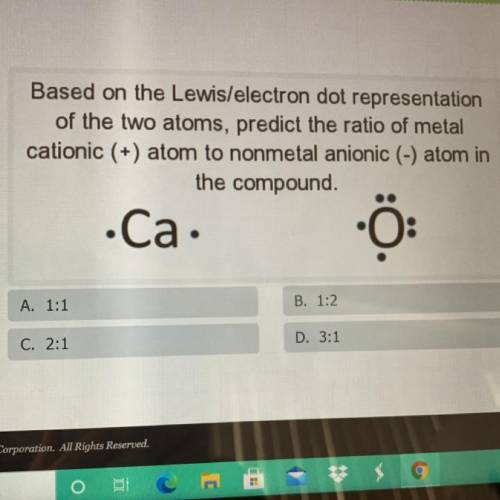
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
c...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
Questions

Chemistry, 20.09.2020 06:01


History, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01


Geography, 20.09.2020 06:01


Mathematics, 20.09.2020 06:01


Mathematics, 20.09.2020 06:01

Social Studies, 20.09.2020 06:01

Social Studies, 20.09.2020 06:01



Biology, 20.09.2020 06:01


Biology, 20.09.2020 06:01