8
с
Which of the following statements is FALSE about the diagrams above?
Diagram B is a...

Chemistry, 17.12.2020 23:50 NEUROPHARMACOLOGICAL
8
с
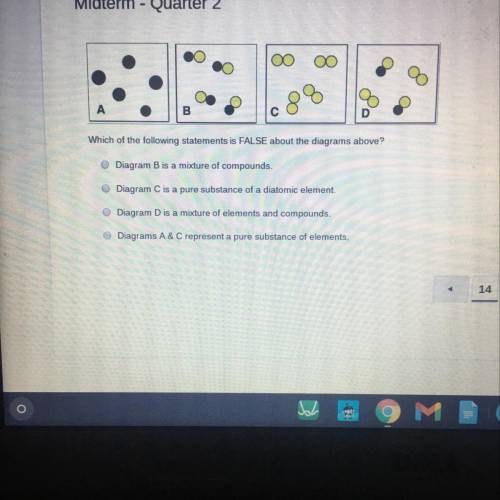
Which of the following statements is FALSE about the diagrams above?
Diagram B is a mixture of compounds.
Diagram C is a pure substance of a diatomic element.
Diagram D is a mixture of elements and compounds.
Diagrams A&C represent a pure substance of elements.
14 15

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2


Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
Questions


Chemistry, 07.11.2020 04:50




Mathematics, 07.11.2020 04:50

Computers and Technology, 07.11.2020 04:50


Mathematics, 07.11.2020 04:50



History, 07.11.2020 04:50

Mathematics, 07.11.2020 04:50


Mathematics, 07.11.2020 04:50


Mathematics, 07.11.2020 04:50



Mathematics, 07.11.2020 04:50



