
Chemistry, 18.12.2020 02:10 itsstaytay200
GIVING BRAINLISTED
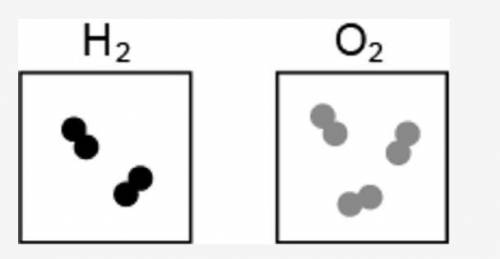
The image represents the reaction between a certain number of molecules of H2 and O2.
Two squares are shown. Inside the left square two units of two joint circles if shown. The molecular formula of hydrogen gas is shown on top of this square. The square on the right has three units of two joint circles. The molecular formula for oxygen gas is shown on top of this square
If the maximum amount of H2O is formed during the reaction, what is the leftover reactant?
One molecule of H2
Two molecules of H2
One molecule of O2
Two molecules of O2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1
You know the right answer?
GIVING BRAINLISTED
The image represents the reaction between a certain number of molecules of H2 an...
Questions

Physics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00


Mathematics, 18.12.2020 01:00






History, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

French, 18.12.2020 01:00


Computers and Technology, 18.12.2020 01:00

Health, 18.12.2020 01:00

World Languages, 18.12.2020 01:00






