REALLY DONT BE THAT GUY

Chemistry, 18.12.2020 03:50 Makoshark6887
GIVING BRAIBLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
In the following reaction, oxygen is the excess reactant.
SiCl4 + O2 → SiO2 + Cl2
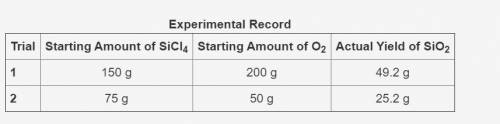
The table shows an experimental record for the above reaction.
-> THE TABLE IS IN THE IMAGE <-
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work.
Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


You know the right answer?
GIVING BRAIBLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
REALLY DONT BE THAT GUY
Questions

Mathematics, 09.03.2021 18:20

Chemistry, 09.03.2021 18:20

Mathematics, 09.03.2021 18:20


Business, 09.03.2021 18:20

Advanced Placement (AP), 09.03.2021 18:20


Mathematics, 09.03.2021 18:20

Chemistry, 09.03.2021 18:20

Biology, 09.03.2021 18:20

Social Studies, 09.03.2021 18:20


Business, 09.03.2021 18:20



Mathematics, 09.03.2021 18:20


Chemistry, 09.03.2021 18:20

Mathematics, 09.03.2021 18:20

Advanced Placement (AP), 09.03.2021 18:20



