Experiment Nine
The Specific Heat of a Metal
DATA:
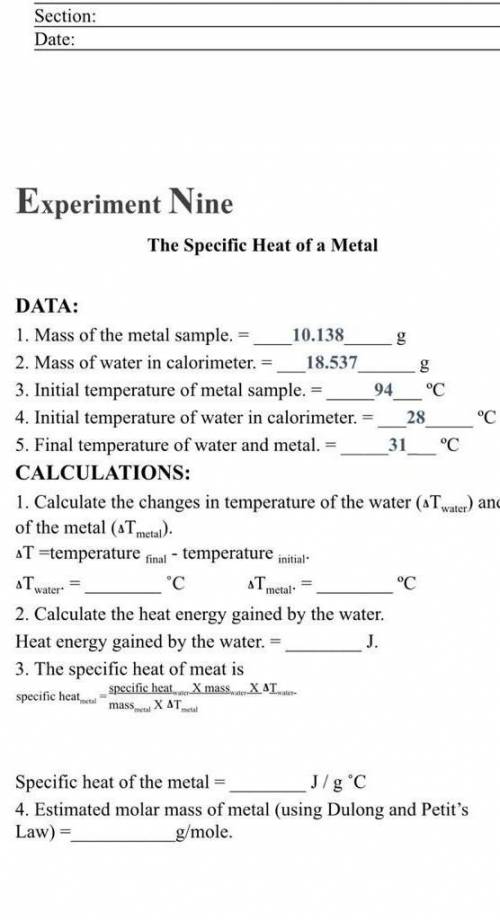
1. Mass of the metal sample.
10.1...

Chemistry, 18.12.2020 04:10 eylinglez3ovm16v
Experiment Nine
The Specific Heat of a Metal
DATA:
1. Mass of the metal sample.
10.138
2. Mass of water in calorimeter. 18.537
3. Initial temperature of metal sample. 94 °C
4. Initial temperature of water in calorimeter. 28 °C
5. Final temperature of water and metal. 31___"C
CALCULATIONS:
1. Calculate the changes in temperature of the water (aT water) and
of the metal (ATmetal).
T =temperature final - temperature initial
"С ST
°C
2. Calculate the heat energy gained by the water.
Heat energy gained by the water. =
J.
3. The specific heat of meat is
specific heat mass. XAT.
specific heal
Tnator
metal
mask.
X ΔΤ.
Specific heat of the metal
J/g °C
4. Estimated molar mass of metal (using Dulong and Petit's
Law) =
g/mole.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1


You know the right answer?
Questions


Mathematics, 05.05.2020 11:30

Mathematics, 05.05.2020 11:30


Mathematics, 05.05.2020 11:30




Biology, 05.05.2020 11:30






Social Studies, 05.05.2020 11:30

Physics, 05.05.2020 11:30


Mathematics, 05.05.2020 11:30

English, 05.05.2020 11:30

Mathematics, 05.05.2020 11:30



