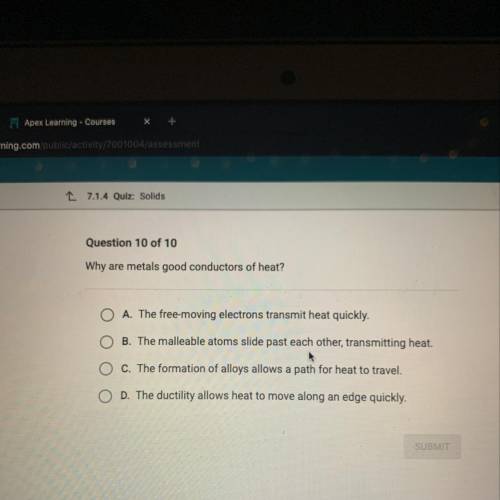
Why are metals good conductors of heat?
O A. The free-moving electrons transmit heat quickly.
...

Chemistry, 18.12.2020 05:50 kerarucker12pe384k
Why are metals good conductors of heat?
O A. The free-moving electrons transmit heat quickly.
B. The malleable atoms slide past each other, transmitting heat.
O c. The formation of alloys allows a path for heat to travel.
O D. The ductility allows heat to move along an edge quickly.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2



Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
Questions

Biology, 29.07.2019 05:30


Computers and Technology, 29.07.2019 05:30


Computers and Technology, 29.07.2019 05:30

History, 29.07.2019 05:30


Arts, 29.07.2019 05:30



Biology, 29.07.2019 05:30

Arts, 29.07.2019 05:30

Mathematics, 29.07.2019 05:30

English, 29.07.2019 05:30


Arts, 29.07.2019 05:30


Arts, 29.07.2019 05:30




