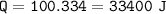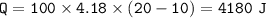Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3


Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
7. Calculate the amount of energy required heat 100.g to the following: a. H2O(s) changes to H2O(l)...
Questions












Mathematics, 23.06.2020 19:01







Mathematics, 23.06.2020 19:01