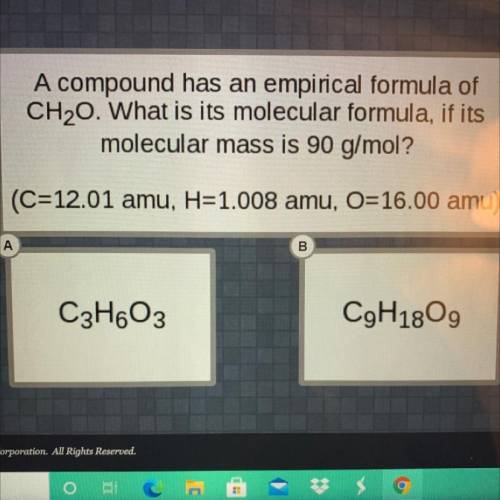
A compound has an empirical formula of
CH,0. What is its molecular formula, if its
molecular...

Chemistry, 18.12.2020 09:10 dragongacha777
A compound has an empirical formula of
CH,0. What is its molecular formula, if its
molecular mass is 90 g/mol?
(C=12.01 amu, H=1.008 amu, O=16.00 amu)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2


Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3
You know the right answer?
Questions





Arts, 05.11.2020 18:40





English, 05.11.2020 18:40


History, 05.11.2020 18:40



Mathematics, 05.11.2020 18:40


Mathematics, 05.11.2020 18:40





