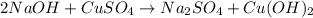Chemistry, 18.12.2020 17:30 Wolfzbayne
You react 14.5 grams of sodium hydroxide with 14.5 grams of copper II sulfate. At the end of the lab you find that you recovered 7.99 grams of precipitate. When you write the balanced equation for you reaction, what is the coefficient in front of sodium hydroxide?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
You react 14.5 grams of sodium hydroxide with 14.5 grams of copper II sulfate. At the end of the lab...
Questions

English, 23.03.2021 19:00

Mathematics, 23.03.2021 19:00

Mathematics, 23.03.2021 19:00

Chemistry, 23.03.2021 19:00

Geography, 23.03.2021 19:00

Biology, 23.03.2021 19:00


History, 23.03.2021 19:00




Biology, 23.03.2021 19:00



History, 23.03.2021 19:00


Mathematics, 23.03.2021 19:00

Mathematics, 23.03.2021 19:00


Mathematics, 23.03.2021 19:00