
Chemistry, 18.12.2020 18:00 esme06quirino
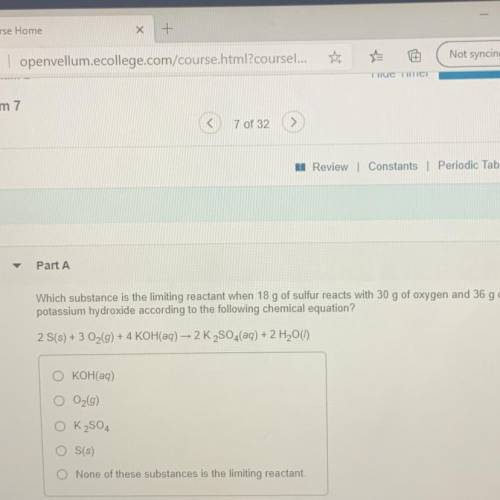
Which substance is the limiting reactant when 18 g of sulfur reacts with 30 g of oxygen and 36 g of
potassium hydroxide according to the following chemical equation?
2 S(s) + 3 O2(g) + 4 KOH(aq) - 2 K2SO4(aq) + 2 H2O(1)
O KOH(aq)
O2(g)
O K2SO4
O S(s)
O None of these substances is the limiting reactant.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

You know the right answer?
Which substance is the limiting reactant when 18 g of sulfur reacts with 30 g of oxygen and 36 g of...
Questions

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

History, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00



English, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Business, 10.03.2021 01:00



Biology, 10.03.2021 01:00

Biology, 10.03.2021 01:00

History, 10.03.2021 01:00


Mathematics, 10.03.2021 01:00


Advanced Placement (AP), 10.03.2021 01:00



