
Chemistry, 19.12.2020 06:00 jsjsjsskakwkowwj
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g)
A) NO increases the rate at which SO3 molecules are formed.
B) NO reacts with SO3 to produce more SO2 molecules.
C) NO decreases collisions between the SO2 and O2 molecules.
D) NO increases the concentration of the SO2 and O2 molecules.
E) NO increases the activation energy of the SO2 and O2 molecules.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1
You know the right answer?
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g...
Questions

Advanced Placement (AP), 27.03.2020 06:10







Biology, 27.03.2020 06:11


Computers and Technology, 27.03.2020 06:11

Mathematics, 27.03.2020 06:11




Spanish, 27.03.2020 06:11


Mathematics, 27.03.2020 06:12


English, 27.03.2020 06:12

Mathematics, 27.03.2020 06:12

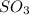 molecules are formed.
molecules are formed.
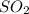 and
and 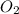 molecules will incraese which in turn will lead to formatioon of more
molecules will incraese which in turn will lead to formatioon of more 

