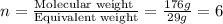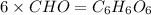Chemistry, 19.12.2020 16:10 amandaestevez030
Burning 12.00 g of an oxoacid produces 17.95 g of carbon dioxide and 4.87 g of water. Consider that 0.25 moles of oxoacid equals 44.0 g. For this compound, determine the empirical and molecular formula.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1
You know the right answer?
Burning 12.00 g of an oxoacid produces 17.95 g of carbon dioxide and 4.87 g of water. Consider that...
Questions


History, 23.07.2020 08:01




Mathematics, 23.07.2020 08:01

Business, 23.07.2020 08:01


Biology, 23.07.2020 09:01

English, 23.07.2020 09:01


Mathematics, 23.07.2020 09:01


Mathematics, 23.07.2020 09:01





Mathematics, 23.07.2020 09:01


 = 17.95 g
= 17.95 g = 4.87 g
= 4.87 g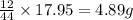 of carbon will be contained.
of carbon will be contained. of hydrogen will be contained.
of hydrogen will be contained.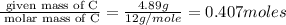
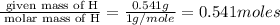
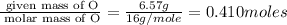
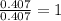


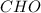 .
.