Chemistry, 19.12.2020 21:50 chriscendypierre56
An oxide of arsenic contains 3.26 g of arsenic and 1.04 g of oxygen. What is the empirical formula for this oxide?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

You know the right answer?
An oxide of arsenic contains 3.26 g of arsenic and 1.04 g of oxygen. What is the empirical formula f...
Questions

Mathematics, 31.03.2021 01:10









Mathematics, 31.03.2021 01:10


Mathematics, 31.03.2021 01:10

Chemistry, 31.03.2021 01:10

Business, 31.03.2021 01:10

Mathematics, 31.03.2021 01:10

Mathematics, 31.03.2021 01:10

Mathematics, 31.03.2021 01:10

Health, 31.03.2021 01:10

Arts, 31.03.2021 01:10

Health, 31.03.2021 01:10

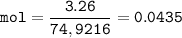
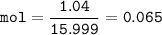 .
.