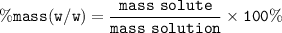Chemistry, 21.12.2020 14:00 snikergrace
Calculate the grams of NaCl (5.5 by mass) in 250 grams of commercial bleach solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Consider the balanced equation below. n2h4 + 2h2o2 n2 + 4h2o what are the mole ratios of hydrazine (n2h4) to hydrogen peroxide (h2o2) and hydrazine to water? 1: 2 and 1: 4 1: 3 and 1: 4 1: 2 and 3: 5 1: 3 and 3: 5
Answers: 3

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
Calculate the grams of NaCl (5.5 by mass) in 250 grams of commercial bleach solution...
Questions


Mathematics, 13.04.2021 20:00



Biology, 13.04.2021 20:00



Biology, 13.04.2021 20:00




History, 13.04.2021 20:00

Mathematics, 13.04.2021 20:00

Chemistry, 13.04.2021 20:00



Chemistry, 13.04.2021 20:00

Physics, 13.04.2021 20:00

Mathematics, 13.04.2021 20:00