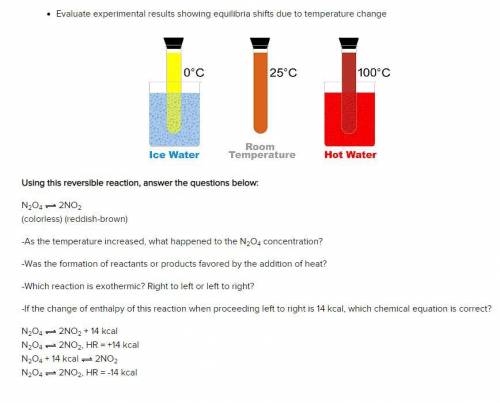
Using this reversible reaction, answer the questions below:
N2O4 2NO2
(colorless) (reddish-br...

Chemistry, 21.12.2020 14:00 mommer2019
Using this reversible reaction, answer the questions below:
N2O4 2NO2
(colorless) (reddish-brown)
-As the temperature increased, what happened to the N2O4 concentration?
-Was the formation of reactants or products favored by the addition of heat?
-Which reaction is exothermic? Right to left or left to right?
-If the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct?
N2O4 2NO2 + 14 kcal
N2O4 2NO2, HR = +14 kcal
N2O4 + 14 kcal 2NO2
N2O4 2NO2, HR = -14 kcal

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Questions

History, 03.12.2021 22:20


Chemistry, 03.12.2021 22:20


Geography, 03.12.2021 22:20








Mathematics, 03.12.2021 22:20

Mathematics, 03.12.2021 22:20










