
Chemistry, 21.12.2020 16:50 Krazyyykiddd
Dilute hydrochloric acid was titrated with sodium carbonate solution. • 10.0 cm3 of 0.100 mol / dm3 hydrochloric acid were placed in a conical flask. • A few drops of methyl orange indicator were added to the dilute hydrochloric acid. • The mixture was titrated with sodium carbonate solution. • 16.2 cm3 of sodium carbonate solution were required to react completely with the acid. What colour would the methyl orange indicator be in the hydrochloric acid? Calculate how many moles of hydrochloric acid were used. Use your answer to (b)(ii) and the equation for the reaction to calculate the number of moles of sodium carbonate that reacted.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 07:30
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1

Chemistry, 23.06.2019 12:50
Acertain reaction has a activation energy of 54.0 kj/mol. as the temperature is increased from 22c to a higher temperature, the rate constant increases by a factor of 7.00. calculate the higher temperature. c (report only numerical answer)
Answers: 3
You know the right answer?
Dilute hydrochloric acid was titrated with sodium carbonate solution. • 10.0 cm3 of 0.100 mol / dm3...
Questions



English, 15.12.2021 23:20


Mathematics, 15.12.2021 23:20

Biology, 15.12.2021 23:20

Business, 15.12.2021 23:20

Mathematics, 15.12.2021 23:20



Mathematics, 15.12.2021 23:20

Mathematics, 15.12.2021 23:20

History, 15.12.2021 23:20

History, 15.12.2021 23:20



Biology, 15.12.2021 23:20


English, 15.12.2021 23:20

Mathematics, 15.12.2021 23:20

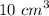 of a 0.100
of a 0.100 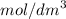 of hydrocholric acid is used.
of hydrocholric acid is used.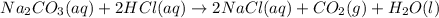
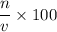
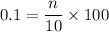
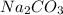 , two moles of HCl acid is used.
, two moles of HCl acid is used.

