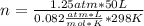Chemistry, 21.12.2020 17:40 deandrebryant89
Automobile air bags inflate during a crash or sudden stop by the rapid generation of nitrogen gas from sodium azide. 2NaN3(s) -->2Na(s) + 3N2 (g)How many moles of sodium azide are needed to produce sufficient nitrogen to fill a 50.0 L air bag to a pressure of 1.25 atm at 25C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2


Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Automobile air bags inflate during a crash or sudden stop by the rapid generation of nitrogen gas fr...
Questions

Mathematics, 21.05.2021 16:20


Mathematics, 21.05.2021 16:20


Mathematics, 21.05.2021 16:20

Social Studies, 21.05.2021 16:20




Mathematics, 21.05.2021 16:20

Mathematics, 21.05.2021 16:20



Mathematics, 21.05.2021 16:20

Mathematics, 21.05.2021 16:20



Physics, 21.05.2021 16:20

Mathematics, 21.05.2021 16:20

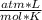 T= 25 C= 298 K
T= 25 C= 298 K