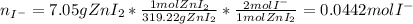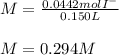Chemistry, 21.12.2020 17:30 aidenbender06
Suppose 7.05 g of zinc iodide is dissolved in 150. mL of a 0.20M aqueous solution of potassium carbonate. Calculate the final molarity of iodide anion in the solution. You can assume the volume of the solution doesn't change when the zinc iodide is dissolved in it. Round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
Suppose 7.05 g of zinc iodide is dissolved in 150. mL of a 0.20M aqueous solution of potassium carbo...
Questions



Mathematics, 15.10.2019 21:50

Social Studies, 15.10.2019 21:50



Mathematics, 15.10.2019 21:50

Biology, 15.10.2019 21:50

Computers and Technology, 15.10.2019 21:50

Mathematics, 15.10.2019 21:50



Biology, 15.10.2019 21:50


Mathematics, 15.10.2019 21:50





Mathematics, 15.10.2019 21:50