
Chemistry, 21.12.2020 17:50 krishawnnn
A voltaic cell is constructed that is based on the following reaction:
Sn2+(aq)+Pb(s)→Sn(s)+Pb2+(aq).
If the concentration of Sn2+ in the cathode compartment is 1.00 M and the cell generates an emf of 0.16 V , what is the concentration of Pb2+ in the anode compartment?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3


Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1
You know the right answer?
A voltaic cell is constructed that is based on the following reaction:
Sn2+(aq)+Pb(s)→Sn(s)+Pb2+(a...
Questions

Mathematics, 12.05.2021 21:30

Mathematics, 12.05.2021 21:30

Arts, 12.05.2021 21:30


Mathematics, 12.05.2021 21:30

Mathematics, 12.05.2021 21:30

Health, 12.05.2021 21:30


Mathematics, 12.05.2021 21:30

English, 12.05.2021 21:30




Mathematics, 12.05.2021 21:30


Biology, 12.05.2021 21:30

English, 12.05.2021 21:30

Mathematics, 12.05.2021 21:30


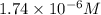

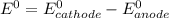
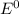 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Sn^{2+}/Sn]}=-0.14V](/tpl/images/1004/1474/81a51.png)
![E^0_{[Pb^{2+}/Pb]}=-0.13V](/tpl/images/1004/1474/82211.png)
![E^0=E^0_{[Sn^{2+}/Sn]}- E^0_{[Pb^{2+}/Pb]}](/tpl/images/1004/1474/cb409.png)
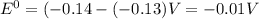
![E=E^0-\frac{0.059}{n}\log\frac{[Pb^{2+}]}{[Sn^{2+}]}](/tpl/images/1004/1474/ec777.png)
![0.16=(-0.01)-\frac{0.059}{2}\log\frac{[Pb^{2+}]}{[1.00]}](/tpl/images/1004/1474/70ccb.png)
![0.17=-0.0295\log\frac{[Pb^{2+}]}{[1.00]}](/tpl/images/1004/1474/5042d.png)
![-5.76=\log\frac{[Pb^{2+}]}{[1.00]}](/tpl/images/1004/1474/a4415.png)
![1.74\times 10^{-6}=\frac{[Pb^{2+}]}{[1.00]}](/tpl/images/1004/1474/3d2ba.png)
![[Pb^{2+}]=1.74\times 10^{-6}](/tpl/images/1004/1474/109b6.png)


