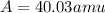Chemistry, 22.12.2020 06:40 91miketaylor
Two isotopes of nickel have a percent abundance of 0.93% and 68.08% with masses of 63.93
amu and 57.93 amu respectively. What is the atomic mass of these two isotopes? Explain why
the average mass is closer to actual amu of 58.69 for nickel.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1


Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
Two isotopes of nickel have a percent abundance of 0.93% and 68.08% with masses of 63.93
amu and 57...
Questions


Physics, 19.12.2019 22:31

Computers and Technology, 19.12.2019 22:31

History, 19.12.2019 22:31














Biology, 19.12.2019 22:31


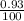
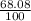
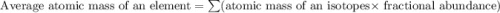
![A=\sum[(63.93)\times \frac{0.93}{100})+(57.93)\times \frac{68.08}{100}]]](/tpl/images/1005/1681/01772.png)