
Chemistry, 22.12.2020 08:40 anaroles04
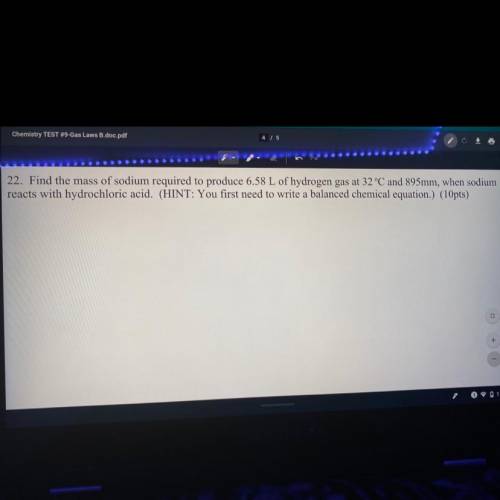
Find the mass of sodium required to reduce 6.58 L of hydrogen gas at 32°C and 895 mm, when sodium reacts with hydrochloric acid. (Hint: you first need to write a balanced chemical equation.)

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Find the mass of sodium required to reduce 6.58 L of hydrogen gas at 32°C and 895 mm, when sodium re...
Questions

Mathematics, 26.10.2020 14:00




Mathematics, 26.10.2020 14:00


English, 26.10.2020 14:00

History, 26.10.2020 14:00

Medicine, 26.10.2020 14:00

English, 26.10.2020 14:00

Mathematics, 26.10.2020 14:00


Biology, 26.10.2020 14:00

History, 26.10.2020 14:00


Mathematics, 26.10.2020 14:00


English, 26.10.2020 14:00

Chemistry, 26.10.2020 14:00

History, 26.10.2020 14:00



