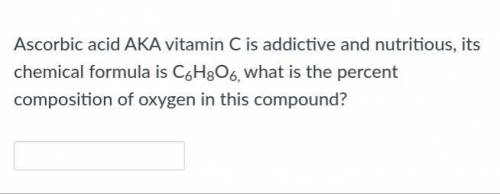
Please answer for me.
...

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Questions


Mathematics, 24.12.2020 14:00


Mathematics, 24.12.2020 14:00


Social Studies, 24.12.2020 14:00

English, 24.12.2020 14:00


English, 24.12.2020 14:00

Social Studies, 24.12.2020 14:00

Mathematics, 24.12.2020 14:00



Computers and Technology, 24.12.2020 14:00

Business, 24.12.2020 14:00


Mathematics, 24.12.2020 14:00

Mathematics, 24.12.2020 14:00

Mathematics, 24.12.2020 14:00