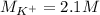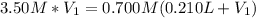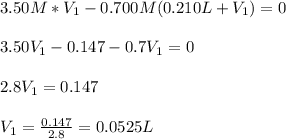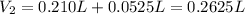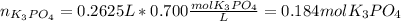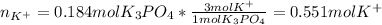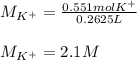Chemistry, 23.12.2020 04:30 neekobecky599
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water to form a 0.700 M K3PO, solution. Calculate the molarity of potassium ions, K in the solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

You know the right answer?
(b) An unknown volume of 3.50 M potassium phosphate, K, PO, solution is added to 0.210 L of water
t...
Questions








Mathematics, 20.10.2021 02:50





English, 20.10.2021 03:00

Chemistry, 20.10.2021 03:00


English, 20.10.2021 03:00

History, 20.10.2021 03:00

English, 20.10.2021 03:00

History, 20.10.2021 03:00

Spanish, 20.10.2021 03:00