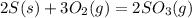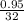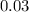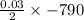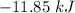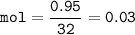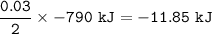Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
The value of ΔH° for the reaction below is -790 kJ. The enthalpy change accompanying the reaction of...
Questions

English, 08.11.2020 14:00

Computers and Technology, 08.11.2020 14:00



Arts, 08.11.2020 14:00

English, 08.11.2020 14:00


Computers and Technology, 08.11.2020 14:00


English, 08.11.2020 14:00




Mathematics, 08.11.2020 14:00



Chemistry, 08.11.2020 14:00



Mathematics, 08.11.2020 14:00