

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1


Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
1.72 moles of NOCI were placed in a 2.50 L reaction chamber
at 427°C. After equilibrium was reached...
Questions




English, 21.12.2021 06:00


Mathematics, 21.12.2021 06:00





Biology, 21.12.2021 06:00

Arts, 21.12.2021 06:00

Business, 21.12.2021 06:00


English, 21.12.2021 06:00




Mathematics, 21.12.2021 06:00

Mathematics, 21.12.2021 06:00

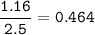
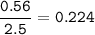
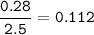
![\tt Kc=\dfrac{[NO]^2[Cl_2]}{[NOCl]^2}\\\\Kc=\dfrac{0.224^2\times 0.112}{0.464^2}=0.026](/tpl/images/1007/8914/ad1b4.png)


